Updates to the DUTCH Report
Hilary Miller, ND
and Azra Jaferi
This spring, the DUTCH Test is getting a makeover, with a fresh look and additional features. While you will still find the classic DUTCH Dials and all the biomarkers you are used to, we hope this fresh look will enhance the readability of our report. In addition to changes in the overall visuals, we have four new features:
- Age-dependent ranges integrated into the androgen dials
- The addition of a 5a-Androstanediol dial on the Summary page (an important biomarker of tissue androgens)
- Expanded reporting of androgen biomarkers on the female Sex Hormone page
- The Cortisol Clearance Rate slider, a measure of glucocorticoid metabolism
- Remodeled comments section in the back of the report
This article is a walkthrough of these new features and how they will enhance your use of the DUTCH test.
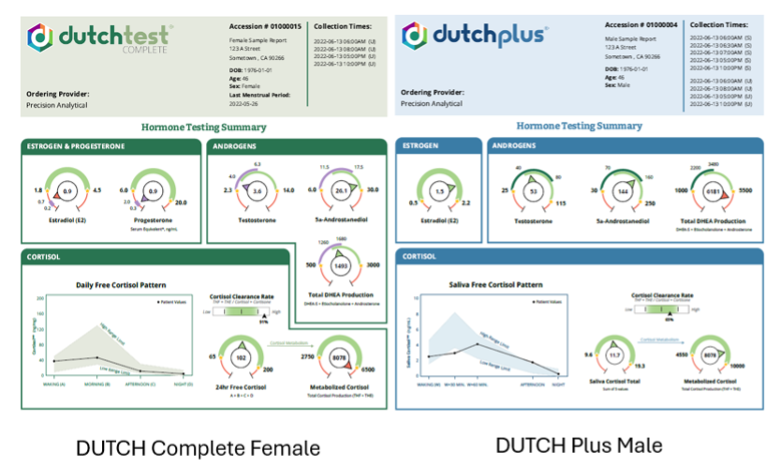
In the examples above, you can see the new DUTCH Complete (left, female example) and the new DUTCH Plus (right, male example) Summary pages. Most of the familiar dials are here to stay, and we have added new androgen dials, the 5a-androstanediol dial, and the Cortisol Clearance Rate slider to the Summary page. With these added features, you will quickly be able to achieve a more complete assessment of your patient's results just by looking at the Summary page.
Androgen Dials Now Show Age-Dependent Ranges
The androgen dials throughout the report now feature color-coded age-dependent ranges with color-coded arrows that point to the point in the ranges that the patient’s result falls. Previously, we communicated age-dependent ranges via a table near the dials. This required finding the patient’s age range and mentally applying it to the patient’s result for each androgen. Now, with little orientation, the dial can be read and understood quickly without having to reference other resources.
In men, the most significant changes in testosterone happen around age 40, therefore, we have applied two ranges which cover 18-40 (light green) and 41+ (dark green). In the example below, this 36-year-old man is below range for his age, clearly shown by the arrow in the dark green and below the light green range. Without the age-dependent ranges, this result might appear to be normal (unless the provider referred to the table).
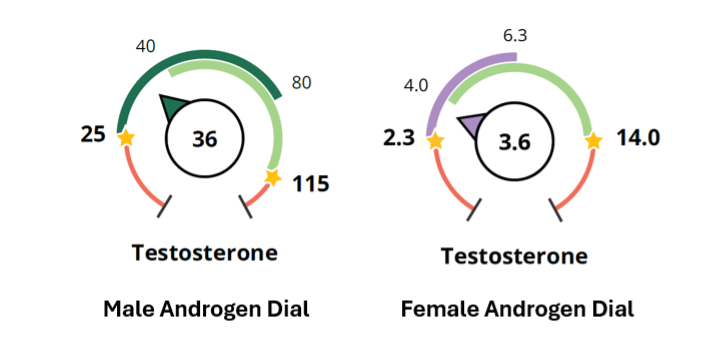
In women, we continue to orient the female sex hormone ranges around optimal androgen levels in premenopause (light green, women 18-40) and postmenopause (purple, women over 56). Women aged 41-55 may have androgens within the premenopausal or postmenopausal range. This will allow you to compare your perimenopausal patients’ androgen levels to both pre- and postmenopausal women to get a better assessment of her status. The androgen dials use the same purple to show postmenopause as on the progesterone and estrogen dials. In the example for female testosterone above, this 58-year-old patient’s testosterone is within her age-dependent range. It is typical to see postmenopausal women in the purple range.
5α-Androstanediol: A Marker of Androgen Status in Women
DUTCH reports have long provided test results on androgens in both men and women. This profiling includes a total of eight different androgen markers (DHEA-S, testosterone, 5α-DHT, 5α-androstanediol, and others), offering a comprehensive picture of androgen production and metabolic patterns. The use of these markers continues to be backed by research. For 5α-androstanediol in particular, the research in women has been growing. Numerous studies are now supporting the value of 5α-androstanediol as a marker of androgen status in women, which prompted an update to DUTCH reports, where providers can now find 5α-androstanediol conveniently showcased on the Summary page.
5α-androstanediol is unique in that it is thought to be the best marker available of DHT formation and activity inside the cell.(1, 2) Having a sensitive marker of DHT activity is clinically relevant because DHT is three times more potent than testosterone in both women and men and acts primarily inside the cells of peripheral tissues such as skin and adipose tissue. For example, in the skin, free (protein unbound) testosterone enters the cells and converts to DHT by the enzyme 5α-reductase. DHT then activates the androgen receptor inside the cell, ultimately leading to gene transcription that regulates hair growth, sebum production, and other physiological effects in the skin. The DHT produced inside this cell remains inside the cell, exerting localized androgenic activity at the tissue level without being released into the blood. Essentially, the most biologically meaningful effects of DHT are through its intracellular actions, which cannot be captured by measuring directly, whether serum or urine. DHT does, however, convert to its metabolite — 5α-androstanediol — which exits the cell into the bloodstream and is then conjugated to a water-soluble form excreted in urine, where it can be measured.
As a marker of intracellular DHT metabolism, 5α-androstanediol has been examined in many studies looking at elevated androgen levels in women and their associated symptoms and conditions. Our forthcoming DUTCH White Paper on 5α-androstanediol provides a more detailed review of the research. Here are some key takeaways:
- 5α-androstanediol levels are abnormally elevated in the blood and urine of women with androgen excess symptoms, including male-pattern hair growth (idiopathic hirsutism), (3-12) scalp hair loss (androgenetic alopecia), (13-17) acne, (2, 18) menstrual irregularities, (17, 19) and PCOS. (20-23)
- 5α-androstanediol levels can be elevated even when testosterone levels are within normal range, as in the case of idiopathic hirsutism in women. (3-12)
- Elevations in 5α-androstanediol levels resolve along with symptom improvement following treatment of excess androgen symptoms in women. (7, 24-28)
- 5α-androstanediol may be a more sensitive marker of androgenic effects in women than other commonly used androgen markers. (9, 20)
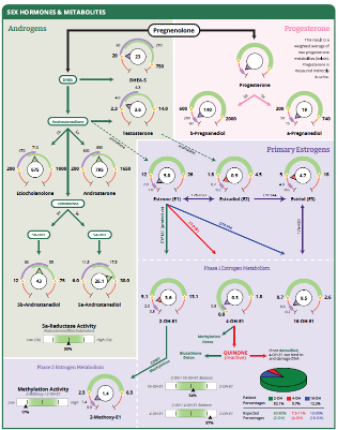
To further enhance the utility of androgens and metabolites, the female Sex Hormone page will show additional androgen metabolite dials, 5a-androstanediol and 5b-androstanediol. These metabolites have always been tested and listed on page 2 of the female DC and DP reports but will now be displayed in dials on page 3 for easier assessment. Please note the male Sex Hormone page has always shown these dials.
Cortisol Clearance: Tissue Regulation of Glucocorticoid Action
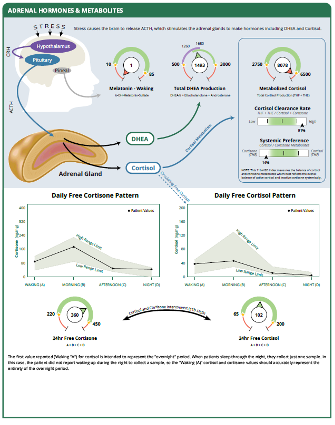
The DUTCH Test measures urinary (DUTCH Complete) or salivary (DUTCH Plus) free cortisol and free cortisone at multiple time points to map the diurnal rhythm of cortisol. In addition, the DUTCH test measures the endogenously formed metabolites of free cortisol and free cortisone, labeled Metabolized Cortisol. These include a-Tetrahydrocortisol (a-THF), b-Tetrahydrocortisol (b-THF), and b-Tetrahydrocortisone (b-THE). Due to the relatively rapid metabolization of cortisol, the metabolized cortisol across the four dried urine samples represents total cortisol production for the day. In 2020, the DUTCH 4-spot dried urine collection was shown in peer-reviewed published data to be comparable to 24-hour urine collections for free and metabolized cortisol measurements (29). Further, in 2023, DUTCH 4-spot urine data found that cortisol metabolite concentrations may aid in identifying the relationship between the HPA axis and body mass index (BMI)(30).
Now the DUTCH Test is introducing a new ratio using the biomarkers we have been analyzing for years. This new slider bar shown on the Summary page and the Adrenal page, is labeled Cortisol Clearance Rate. The Cortisol Clearance Rate is calculated by dividing the total metabolites of cortisol and cortisone (THF + THE, a.k.a "Metabolized Cortisol") by the free cortisol + free cortisone total.

“Cortisol clearance” describes the body’s rate of breakdown and removal of cortisol via 5a and 5b-reductases. 5b-reductase is a liver enzyme but is also expressed in adipose (body fat). 5α-reductase is present in the liver but also present in target tissues such as skin, brain, muscle, and adipose. The liver plays a key role in cortisol breakdown, making it an important regulator of glucocorticoid action in the body. However, the 5a and 5b-reductases can be enhanced or inhibited by conditions such as inflammation, insulin resistance, obesity, and thyroid hormone imbalance,making Cortisol Clearance a potential marker of the impact of these conditions on the HPA axis and overall health.
An important driver of 5a and 5b-reductase enzyme transcription is thyroid hormone. Thyroid hormone receptor activation on genes encoding 5a and 5b-reductase leads to increased mRNA levels and enzyme transcription(31). However, 5a and 5b-reductase is overexpressed in obese patients in both the liver and adipose tissue, promoted by insulin. The chief contributors to cortisol metabolism are thyroid hormone, liver function, obesity, and insulin.
Fast cortisol clearance occurs with elevated levels of 5a and 5b-reductase. This occurs most commonly in obesity but can also be seen with hyperthyroidism or too much thyroid medication. In most cases of fast cortisol clearance, the HPA axis maintains free cortisol at normal circulating levels despite rapid breakdown (30). However, there are times when cortisol production does not “keep up” with cortisol metabolism, leaving the patient with low cortisol, fatigue, sluggish immune function, and lacking the potentially beneficial metabolic effects of cortisol. Additionally, enhanced cortisol metabolism causes hypersecretion of ACTH from the hypothalamic-pituitary complex, which can lead to high adrenal androgen excretion(32).
Slow cortisol clearance occurs with low levels of 5a and 5b-reductase, which can be caused by inadequate thyroid hormone. It is also seen in cholestasis because 5a and 5b-reductase are inhibited by bile acids. When bile acids back up or remain in the liver, cortisol metabolism can slow down. Slow cortisol clearance is also seen with critical conditions such as severe anorexia (likely due to hypothyroid adaptation), critical illness, and liver cirrhosis. Like fast cortisol clearance, the HPA axis usually slows down to maintain normal levels of free cortisol, which can lead to low adrenal androgen output, which is especially impactful on global androgen levels in women. However, free cortisol can “back up,” leading to high cortisol levels, especially during a stress response, when the body is trying to raise cortisol levels rapidly. Subsequently, one common finding with slow cortisol clearance is high morning free cortisol.
To summarize:
Fast cortisol clearance is seen with:
- Obesity (33-35)
- High insulin or insulin resistance(34, 36, 37)
- Hyperthyroidism(38)
- Fatty liver disease

Slow cortisol clearance is seen with:
- Hypothyroidism(38-40)
- Severe anorexia nervosa(41)
- Cholestasis or sluggish removal of bile acids(42)
- Liver cirrhosis (30)

The Adrenal page will show a new cortisol metabolism box, which you can see in the upper right-hand corner. It includes the assessment tools we have always shown, Metabolized Cortisol and the Systemic Preference (11b-HSD index) slider, along with the new Cortisol Clearance Rate slider.
Changes to the Clinical Support Overview
The final feature we have added to the new DUTCH report is a remodeled Clinical Support Overview. This section at the end of the report has historically contained a detailed explanation of the DUTCH analytes, including relevant physiology to orient the reader to the test. It also contained comments that were specifically related to the testing results and patient.
When the DUTCH Test started, we did not have any resources for patients and providers besides the actual report they received. The original Clinical Support Overview was born of a need to explain why urine is uniquely useful for assessing hormones and metabolism. The logical result of this was to add a lot of information to the back of the report, ensuring everyone who ordered a test had this resource in hand. Unfortunately, this may have had a negative impact: The physiology lessons and lengthy explanations became a barrier to providers consistently reading the patient-specific comments.
Over the years, DUTCH educational resources have grown immensely, and these resources far outreach the scope of the Clinical Support Overview. Because of how much these resources cover, we have refined the Clinical Support Overview in the back of the report to focus on the specific patient’s results, relevant medications, and any lab comments that can aid in reading the test.
We at DUTCH are so excited to introduce these new features to our test. The fresh look is only one small part of the report. As you start receiving new DUTCH reports, remember that the core measurements are still the same. DUTCH has made these assessments easier to visualize and teach to your patients so they can better understand this comprehensive assessment.
References:
1. Horton R. Dihydrotestosterone is a peripheral paracrine hormone. J Androl. 1992;13(1):23-27.
2. Lookingbill DP, et al. Tissue production of androgens in women with acne. J Am Acad Dermatol. 1985;12(3):481-487.
3. Falsetti L, et al. Serum levels of 3alpha-androstanediol glucuronide in hirsute and non hirsute women. Eur J Endocrinol. 1998;138(4):421-424.
4. Gompel A, et al. Contribution of plasma androstenedione to 5 alpha-androstanediol glucuronide in women with idiopathic hirsutism. J Clin Endocrinol Metab. 1986;62(2):441-444.
5. Greep N, et al. Androstanediol glucuronide plasma clearance and production rates in normal and hirsute women. J Clin Endocrinol Metab. 1986;62(1):22-27.
6. Horton R, et al. 3 alpha, 17 beta-androstanediol glucuronide in plasma. A marker of androgen action in idiopathic hirsutism. Journal of Clinical Investigation. 1982;69(5):1203-1206.
7. Kirschner MA, et al. Clinical usefulness of plasma androstanediol glucuronide measurements in women with idiopathic hirsutism. J Clin Endocrinol Metab. 1987;65(4):597-601.
8. Mauvais-Jarvis P, et al. Simultaneous determination of urinary androstanediol and testosterone as an evaluation of human androgenicity. J Clin Endocrinol Metab. 1973;36(3):452-459.
9. Muller LM, et al. Urinary 5 alpha-androstane-3 alpha,17 beta-diol levels in normal and hirsute women: discriminating power and relation to other urinary steroids. J Steroid Biochem. 1988;31(6):979-982.
10. Samojlik E, et al. Elevated production and metabolic clearance rates of androgens in morbidly obese women. J Clin Endocrinol Metab. 1984;59(5):949-954.
11. Toscano V, et al. Two different pathogenetic mechanisms may play a role in acne and in hirsutism. Clin Endocrinol (Oxf). 1993;39(5):551-556.
12. Toscano V, et al. Is 3 alpha-androstanediol a marker of peripheral hirsutism? Acta Endocrinol (Copenh). 1982;99(2):314-320.
13. Chen W, et al. Cutaneous Androgen Metabolism: Basic Research and Clinical Perspectives. Journal of Investigative Dermatology. 2002;119(5):992-1007.
14. De Villez RL, et al. Female androgenic alopecia. The 3 alpha,17 beta-androstanediol glucuronide/sex hormone binding globulin ratio as a possible marker for female pattern baldness. Arch Dermatol. 1986;122(9):1011-1015.
15. Legro RS, et al. Alterations in androgen conjugate levels in women and men with alopecia. Fertil Steril. 1994;62(4):744-750.
16. Montalto J, et al. Plasma C19 steroid sulphate levels and indices of androgen bioavailability in female pattern androgenic alopecia. Clin Endocrinol (Oxf). 1990;32(1):1-12.
17. Vexiau P, et al. Role of androgens in female-pattern androgenetic alopecia, either alone or associated with other symptoms of hyperandrogenism. Arch Dermatol Res. 2000;292(12):598-604.
18. Cunha MG, et al. Acne in adult women and the markers of peripheral 3 alpha-diol G activity. J Cosmet Dermatol. 2016;15(4):330-334.
19. Khoury MY, et al. Serum levels of androstanediol glucuronide, total testosterone, and free testosterone in hirsute women. Fertil Steril. 1994;62(1):76-80.
20. Dhayat NA, et al. Urinary steroid profiling in women hints at a diagnostic signature of the polycystic ovary syndrome: A pilot study considering neglected steroid metabolites. PLOS ONE. 2018;13(10):e0203903.
21. Fassnacht M, et al. Beyond adrenal and ovarian androgen generation: Increased peripheral 5 alpha-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(6):2760-2766.
22. Lobo RA. Disturbances of androgen secretion and metabolism in polycystic ovary syndrome. Clin Obstet Gynaecol. 1985;12(3):633-647.
23. Saito K, et al. Steroidogenic pathways involved in androgen biosynthesis in eumenorrheic women and patients with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2016;158:31-37.
24. Carmina E, et al. Time-dependent changes in serum 3 alpha-androstanediol glucuronide correlate with hirsutism scores after ovarian suppression. Gynecol Endocrinol. 1995;9(3):215-220.
25. Fruzzetti F, et al. Effects of finasteride, a 5 alpha-reductase inhibitor, on circulating androgens and gonadotropin secretion in hirsute women. J Clin Endocrinol Metab. 1994;79(3):831-835.
26. Lookingbill DP, et al. Effect of isotretinoin on serum levels of precursor and peripherally derived androgens in patients with acne. Arch Dermatol. 1988;124(4):540-543.
27. Moghetti P, et al. Clinical and hormonal effects of the 5 alpha-reductase inhibitor finasteride in idiopathic hirsutism. J Clin Endocrinol Metab. 1994;79(4):1115-1121.
28. Petrone A, et al. Usefulness of a 12-month treatment with finasteride in idiophathic and polycystic ovary syndrome-associated hirsutism. Clin Exp Obstet Gynecol. 1999;26(3-4):213-216.
29. Newman M, et al. Dried urine and salivary profiling for complete assessment of cortisol and cortisol metabolites. J Clin Transl Endocrinol. 2020;22:100243.
30. Newman MS, et al. Comprehensive assessment of cortisol and cortisol metabolites provides insight into the complex relationship between HPA axis function and BMI. Endocrine and Metabolic Science. 2023;13.
31. Singh BK, et al. Novel Transcriptional Mechanisms for Regulating Metabolism by Thyroid Hormone. International Journal of Molecular Sciences. 2018;19(10):3284.
32. Gambineri A, et al. Increased clearance of cortisol by 5beta-reductase in a subgroup of women with adrenal hyperandrogenism in polycystic ovary syndrome. J Endocrinol Invest. 2009;32(3):210-218.
33. Abraham SB, et al. Cortisol, obesity, and the metabolic syndrome: a cross-sectional study of obese subjects and review of the literature. Obesity (Silver Spring). 2013;21(1):E105-117.
34. Baudrand R, et al. Overexpression of hepatic 5α-reductase and 11β-hydroxysteroid dehydrogenase type 1 in visceral adipose tissue is associated with hyperinsulinemia in morbidly obese patients. Metabolism. 2011;60(12):1775-1780.
35. Rask E, et al. Cortisol metabolism after weight loss: associations with 11 beta-HSD type 1 and markers of obesity in women. Clin Endocrinol (Oxf). 2013;78(5):700-705.
36. Baudrand R, et al. Increased urinary glucocorticoid metabolites are associated with metabolic syndrome, hypoadiponectinemia, insulin resistance and beta cell dysfunction. Steroids. 2011;76(14):1575-1581.
37. Tomlinson JW, et al. Impaired glucose tolerance and insulin resistance are associated with increased adipose 11beta-hydroxysteroid dehydrogenase type 1 expression and elevated hepatic 5alpha-reductase activity. Diabetes. 2008;57(10):2652-2660.
38. Hoshiro M, et al. Comprehensive study of urinary cortisol metabolites in hyperthyroid and hypothyroid patients. Clin Endocrinol (Oxf). 2006;64(1):37-45.
39. Iranmanesh A, et al. Dynamics of 24-hour endogenous cortisol secretion and clearance in primary hypothyroidism assessed before and after partial thyroid hormone replacement. J Clin Endocrinol Metab. 1990;70(1):155-161.
40. Vantyghem MC, et al. Urinary cortisol metabolites in the assessment of peripheral thyroid hormone action: Overt and subclinical hypothyroidism. J Endocrinol Invest. 1998;21(4):219-225.
41. Wassif WS, et al. Steroid metabolism and excretion in severe anorexia nervosa: effects of refeeding. Am J Clin Nutr. 2011;93(5):911-917.
42. Petrescu AD, et al. Hypothalamus-Pituitary-Adrenal Dysfunction in Cholestatic Liver Disease. Frontiers in Endocrinology. 2018;9.
43. Boonen E, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368(16):1477-1488.
TAGS
DUTCH Test: Basic Interpretation Resources
Laboratory Testing: In-Depth Look at DUTCH Urine Testing
Androgens (Testosterone/DHEA)
Cortisol